Overseeing Medicare’s new authority to negotiate prices of the country’s highest-spending drugs is among the top priorities for lawmakers as Congress returns to Washington.
Democrats did better than expected in the midterm elections, making it less likely Republicans will follow through on threats to repeal components of the Biden administration’s landmark Inflation Reduction Act, policy analysts say. But lawmakers and industry groups plan to put pressure on the Medicare agency as it prepares to start negotiating drug prices, including by holding congressional oversight hearings and pursuing clarity on the law’s provisions.
“The industry as a whole is at an inflection point,” said Duane Wright, a senior research analyst at Bloomberg Intelligence.
“They’ll be aiming a lot of their firepower at the regulatory process to shape the law, while focusing legislative efforts on other priorities,” including transparency around pharmacy benefit managers—the entities that manage drug coverage for health insurers, large employers, and others, he said.
Generic and biosimilar competition is another area likely to get attention from Congress as lawmakers seek to go beyond the issues addressed in the IRA (Public Law 117-169). Some lawmakers plan to reignite their push to lower what Americans across health insurance plans pay for insulin.
Sen. Bernie Sanders (I-Vt.), who is poised to take over the Senate Health, Education, Labor and Pensions Committee, expressed optimism on what members of his caucus could get done with Democrats holding 51 seats in the Senate—an improvement from the previous 50-50 split. Sanders has pointed to prescription drug prices as a major priority for him in the new year.
“We can move much more aggressively in passing legislation to protect working families,” he said in an interview.
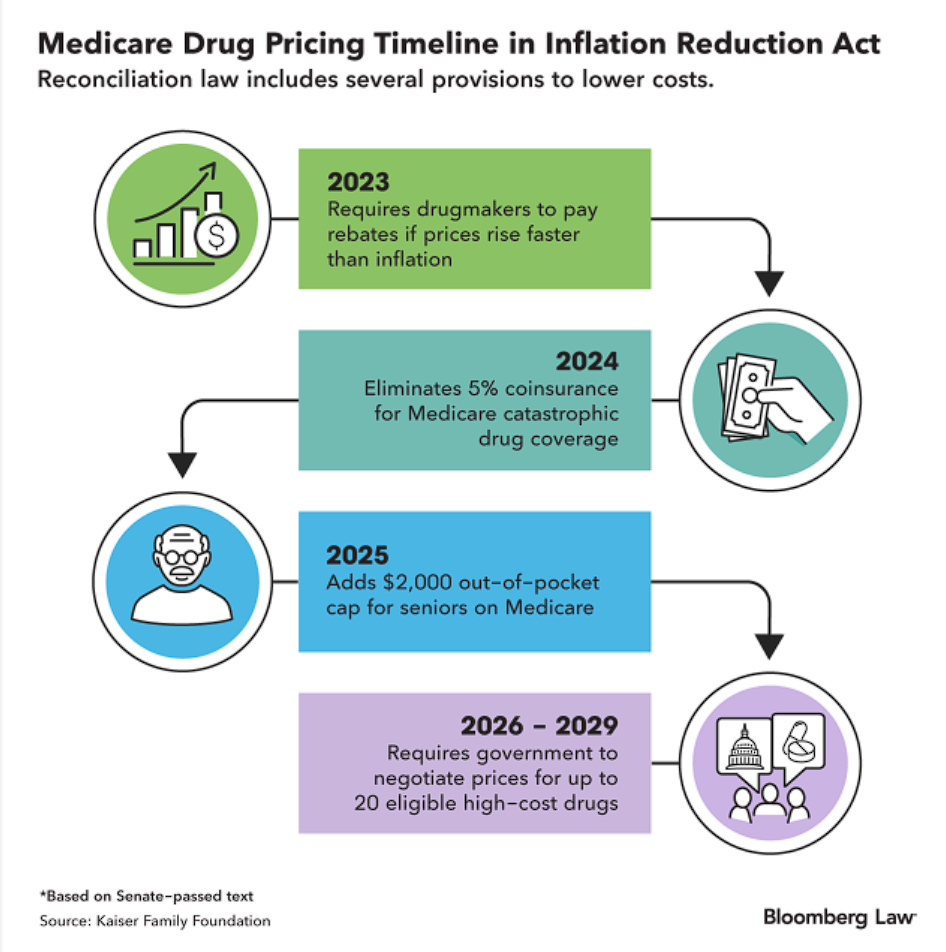
Drug Competition
Republicans and pharmaceutical groups have warned that companies will have less incentive to develop new products, including generics and biosimilars—lower-cost versions of biologics—while trying to compete with government-negotiated drug prices pursuant to the IRA.
They argue that market competition, not government intervention, should help drive down the cost of medicines.
Rep. Cathy McMorris Rodgers (R-Wash.), incoming chair of the House Energy and Commerce Committee, said in an emailed statement that working “to expose the damage done to the generic drug industry by the IRA” is one of her key priorities.
Part of encouraging generic development will require addressing certain patent practices by brand-name drugmakers that allegedly restrict competition, said Anna Kaltenboeck, head of the drug pricing practice at healthcare consulting firm ATI Advisory, who until October served as a senior health policy adviser on the Senate Finance Committee.
“There are instances in which these reference products have patent-thickened themselves to a very high degree and make it very difficult for biosimilars to enter the market within a reasonable time to bring down prices,” she said. In the drug industry, patent thickets refer to when brand-name manufacturers seek multiple patents for minor variations on a single product to extend the period that they’re protected from competition.
PBM Transparency
One of the drug industry’s major complaints against the IRA is that it doesn’t address what they see as the root cause of high drug costs—pharmacy benefit managers and the rebates and fees they collect from drug manufacturers and pharmacies.
Independent pharmacies say PBMs’ recent integration with retail pharmacies and health plans has steered patients away from their businesses, while drug manufacturers argue that having to pay PBMs rebates and discounts results in higher initial list prices.
The Pharmaceutical Care Management Association, the leading PBM trade group, says they haven’t limited the healthy growth of the independent pharmacy marketplace, and that PBMs work to deliver discounts to patients.
Lawmakers, especially Republicans, have proposed legislation that could resurface in the new Congress. A bill (S. 4293) from Sen. Chuck Grassley (R-Iowa) would prohibit PBMs from practicing spread pricing, or charging health plans more for a drug than they reimburse to pharmacies. It would also require PBMs to pass 100% of rebates to health plans, and authorize the Federal Trade Commission and state attorneys general to seek civil penalties for violations of FTC mandates.
PBMs are a top priority for McMorris Rodgers in the new Congress, specifically investigating if “PBMs are keeping lower-cost drugs off formularies in favor of higher rebated brand drugs.”
JC Scott, PCMA’s president, and CEO said in an interview that the group would “have great concern with legislation that would take away choices for employers and other plan sponsors to design their drug benefits in the way to best meet the unique needs of their patient populations.”
Insulin Costs
The insulin cap included in the IRA didn’t go as far as some lawmakers would have liked in working toward achieving wider savings for patients who rely on the drug.
Sen. Jeanne Shaheen (D-N.H.), along with Sen. Susan Collins (R-Maine), announced a bill in the last Congress to limit to $35 per month what people with private insurance pay for diabetes medicine. The bill would also make changes to rebate policies aimed at lowering the actual price of insulin.
Shaheen told Bloomberg Government in December that she planned to renew her proposed insulin cap with Collins in the next Congress, adding that she thinks this could see bipartisan movement.
Overall, the new legislative session presents an opportunity for a better understanding of how drug prices are set and how to combat rising patient costs, Scott said.
“There’s a continued bipartisan interest in issues around prescription drug affordability, and a lot of that will be an opportunity for dialogue, education, and engagement,” he said.
The original article is taken from Bloomberg Law here.


